
Andy Lloyd's Dark Star Blog

Blog 61 (April 2018)
Oxygen production on a Comet
One of the advantages of writing a non-fiction book is
that you can take the opportunity to look back over
previous materials and pick up forgotten threads.
ESA's Rosetta mission to Comet 67P provided a wealth of
scientific data, and some fabulous images of the comet's
rubber duck-shaped nucleus. One of the most
curious findings to emerge from analysis of the vapours
driven off the comet was the unexpected presence of
molecular oxygen (1). Perhaps because we are so
accustomed to the oxygen-rich composition of the Earth's
atmosphere, the presence of gases exuding from Comet 67P
containing 4% oxygen may not seem so weird. But it
really is.
Image Credit: ESA
Oxygen is a reactive molecule, and will go out of its
way to combine with hydrogen to make a much more stable
combination in water. Because there isn't much
hydrogen around in the Earth's atmosphere, oxygen
doesn't have anyone to play with. Besides, our
planet's oxygen supply is continuously replenished by
photosynthesis. No one, not even the wildest
conspiracy theorists, could advocate photosynthesis
going on within a comet. So, there's a problem.
Comets are ancient bodies, left over building blocks
from the very beginning of the solar system. Any
oxygen held by the comet has had both time and
opportunity to combine with hydrogen to make water.
It simply shouldn't be available as a volatile ice,
waiting for the Sun's heat to boil it off into space, as
is the case with water and carbon dioxide. Many
scientists rushed to bang that square peg into a round
hole, but their arguments did not seem particularly
convincing at the time.
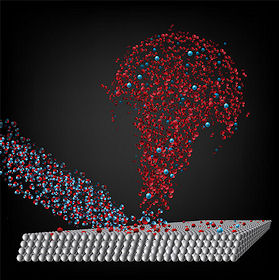
Image Credit: Caltech
Coming to the rescue was a chemical engineer working with silicon superconductor surfaces. He realised that the laboratory work he has been doing for many years had similarities to what might be happening on the surface of a comet. Cometary surfaces contain silicates and residual organic materials aplenty - they are hotbeds for space chemistry (2). Professor Giapis and a research colleague have performed laboratory experiments firing ionised water against silicon-rich surfaces at high speed, and measuring the gaseous materials being blasted off the surface. This analogy with what may be happening on the surface of a comet is neatly captured in their abstract:
"Here we report an original Eley–Rideal reaction mechanism, which permits direct O2 formation in single collisions of energetic water ions with oxidized cometary surface analogues. The reaction proceeds by H2O+ abstracting a surface O-atom, then forming an excited precursor state, which dissociates to produce O2 -. Subsequent photo-detachment leads to molecular O2, whose presence in the coma may thus be linked directly to water molecules and their interaction with the solar wind." (3)
This is how this may be playing out on a comet: Water is driven off the comet by the usual sublimation process: The irradiating heat of the Sun causes the volatile water ices to be converted into vapour. A similar process takes place with dry ice, blasting carbon dioxide into space, and driving out the water vapour through a process known as 'outgassing'. This is, of course, what gives a comet its distinctive tail. Once in space, the irradiating power of the Sun ionises some of the water, and the solar wind drives some of it back down against the surface of the comet. Here, the ionised gas hits materials which have oxygen built into them in the form of oxides, like rust and silicates. The ionised water drives some of this oxygen out, and combines with it to produce molecular oxygen. Wow.

This work interests me for another reason. I'm really interested in what is happening with planets outside the Sun's heliosphere. Beyond this distant boundary in the solar system, objects are technically in interstellar space, even though they may still be orbiting around the Sun. The conditions out there are different. I think it's likely that these different conditions create the potential for new planet-forming mechanisms over long periods of time - or at least, the capacity for unusual effects around planets which would not happen within the heliosphere (4). There are two differences to consider. The first is that the driving solar wind does not extend beyond the heliopause, and the mechanisms for 'clearing' interplanetary space are not at play. The second is that objects beyond the heliosphere are exposed to diffuse interstellar materials, which are moving at high speed comparatively. These include water and ionised water (astrochemistry is assisted by highly energetic cosmic rays).
"It is important to realize ... the option of the direct (Eley−Rideal) process, in which the reactant lands on top of the surface molecule, or the “hot atom” processes in which the reactant reaches the surface with extra translational energy." (5)
This combination could provide some very interesting effects around minor and major bodies beyond the heliosphere, and particularly on and around planets. Planets, after all, have sufficient gravity to be able to hang on to their atmospheres, unlike comets. I will explain more about this in the forthcoming new Dark Star book.
Written by Andy Lloyd, 7th April 2018
References:
1) Andy Lloyd "Oxygen, on a Comet...?" 29 October 2015,
andylloyd.org/darkstarblog31.htm
2) Caltech Press Release "Caltech Chemical Engineer Explains Oxygen Mystery on Comets" 8 May 2017
3) Yunxi Yao and Konstantinos Giapis, "Dynamic molecular oxygen production in cometary comae", Nature Communications, 2017, 8,
4) Andy Lloyd "The Cumulative Effect of Intermittent Interstellar Medium Inundation Upon Objects In The Outer Solar System" February 2016, DOI: 10.13140/RG.2.1.5112.5526
5) Ewine van Dishoeck, Eric Herbst and David A. Neufeld "Interstellar Water Chemistry: From Laboratory to Observations" Chemical Reviews, November 2013
Diamonds from a Lost Planet
Meteorites that fell to Earth in 2008 contain evidence
of a destroyed planet. Following the
explosion in the air of the 4m wide
2008 TC3 asteroid over the Nubian
desert in Sudan, meteorite hunters have since gathered
together 600 meteorite fragments, totalling 10 kg of the
ancient space rock. This came to collectively be
known as the Almahata Sitta
meteorite. Although this quantity of space rock is
impressive, it is only a tiny fraction of the initial
mass of the asteroidal meteor, which had weighed some
80,000kg. This was the first time an asteroid had
been tracked and predicted to impact the Earth (1).
The fireball that resulted from the mid-air explosion of
this bolide was seen hundreds of miles away. The
event was reminiscent of the Tunguska explosion 100
years before, although on a much smaller scale.
Asteroids cut across the Earth's orbital path more often
than people realise (2).
Early analysis of Almahata Sitta
meteorite fragments showed the inclusion of nanodiamond
and graphite micrograins among the more common olivine
grains (3). Some of the nanodiamond fragments were
fractured segments of what had once been larger
diamonds. Impurities within the diamond fragments
indicated that they had grown slowly, rather than having
been formed as a result of a sudden catastrophic
collision. Their relative size implies the same.
The most likely environment for such growth to take
place is deep within the mantle of a planet. Even
a sizeable asteroid may not be large enough to create
these internal conditions. This begs the question:
How could material like this have found its way into
space, having originated deep within a planet?
The early solar system offers the opportunity to place
such an event within a catastrophic milieu. It is
thought that there were a great many proto-planets
forming, and jostling about, during the first ten
million years of the solar system. Some of these
went on to form larger planets; others were ejected into
interstellar space; some may have been destroyed by
internal disruption through the nearby gravitational
influence of a gas giant, like Jupiter; more still were
destroyed by collisions with either the Sun, or with
each other. It is this latter possibility which
may helpfully explain how diamonds from deep inside
planets end up in rocky asteroids milling around in
space. The question is, how big did this disrupted
ancient world need to have been to have formed these
precious internal grains?
New analytical work has found impurities within the
diamonds suggestive of a formation pressure greater than
20 GPa. This, in turn, implies the parent body was
a Mars-sized or Mercury-sized planet, or similarly-sized
planetary embryo (4). Indeed, this may be the
first firm piece of evidence to suggest the prior
existence of early planet-sized embryo in the early
solar system (5).
There are other possibilities, of course. The
Moon-forming event some 4.5 billion years ago most
likely involved a collision between the Earth and a
Mars-sized planet (known as Theia). In the
giant-impact hypothesis, a deep chunk of the Earth was
ripped out into space, and the resultant debris cloud
eventually coalesced into the Moon. The Moon and
Earth share a great deal compositionally.
Recently, it has been realised that the Moon contains
far more water than had been thought possible, and that
it had a wet start to life (6). The Moon also
seems to share exactly the same isotopic composition of
oxygen as the Earth; so much so, that it's hard to
figure out what happened to the impactor in all of this
(7). If the Moon exactly mimics the Earth in its
composition, then where are the remains of Theia?
The asteroids, perhaps? It seems no
coincidence, in this regard, that both the Earth and
Moon seem fundamentally related to asteroids found in
the outer asteroid belt.
In which case, might we wonder whether the near-Earth
asteroid 2008 TC3 had come back to
haunt us?
Written by Andy Lloyd, 19th April 2018


References:
1) BBC News "80-ton asteroid's impact recorded" 25 March 2009,
2) Eddie Irizarry "Asteroid buzzed Earth this weekend" 16 April 2018,
3) Masaaki Miyahara et al. "Unique large diamonds in a ureilite from Almahata Sitta 2008 TC3 asteroid" Geochimica et Cosmochimica Acta, 163, 15 August 2015, pp 14-26
4) Farhang Nabiei et al. "A large planetary body inferred from diamond inclusions in a ureilite meteorite" Nature Communications, 9: 1327
5) Ian Sample "Diamonds in Sudan meteorite 'are remnants of lost planet'" 18 April 2018, with thanks to Al
6) Yanhao Lin et al. "Evidence for an early wet Moon from experimental crystallization of the lunar magma ocean" Nature Geoscience, volume 10, pages 14–18 (2017)
7) Javier Barbuzano "Could a Giant Impact Have Vaporized Earth to Create the Moon?" 1 March 2018,
A Very Entertaining Anunnaki Video
This will no doubt appeal to anyone interested in the writings of Zecharia Sitchin. A word of caution - there's some comic book nudity about 20 minutes in...
Thanks to Jim!

You can keep informed of updates by following me on Twitter:
![]()
Or like my Facebook Page: https://www.facebook.com/darkstarandylloyd